
The hydrated salts of chromium sulfate can also be produced, albeit impure, by extraction of various other chromium compounds, but these routes are not economically viable. Evaporation of these acidic solutions affords the hydrate salt described above. A chromium(III) oxide coproduct is generated which is readily extracted into sulfuric acid. Anthroquinone and quinone are produced on large scale by treatment of anthracene and phenol with chromic acid. The most useful source of chromium(III) sulfate are the Cr(III) wastes from the chromate oxidation of various organic compounds. Structure of Cr(SO 4)(H 2O)(OH), showing the CrO 6 coordination sphere typical of many chromium(III) compounds.

Other chromium(III) hydroxides have been reported. It results from the partial neutralization of the hexahydrates. Most important commercially is basic chromium sulfate, which is thought to be SO 4 (CAS#3). Further heating yields the anhydrous sulfate.Ī variety of other chromium(VI) sulfates are known, but also contain hydroxide or oxide ligands. It is obtained by heating the 18-hydrate material above 70 ☌. Hydrated chromium(III) sulfate, Cr 2(SO 4) 3♱5(H 2O), (CAS #1) is a green solid that also readily dissolves in water.

Six of the eighteen water molecules in this formula unit are water of crystallization. The formula of this compound can be written more descriptively as 2(SO 4) 3♶H 2O. Hydrated chromium(III) sulfate, Cr 2(SO 4) 3♱8H 2O, (CAS #1) is a violet solid that readily dissolves in water to give the metal aquo complex, 3+.Anhydrous chromium(III) sulfate, Cr 2(SO 4) 3, (CAS #1) is a violet solid that dissolves in water upon addition of a reducing agent, which generates chromium(II) sulfates.
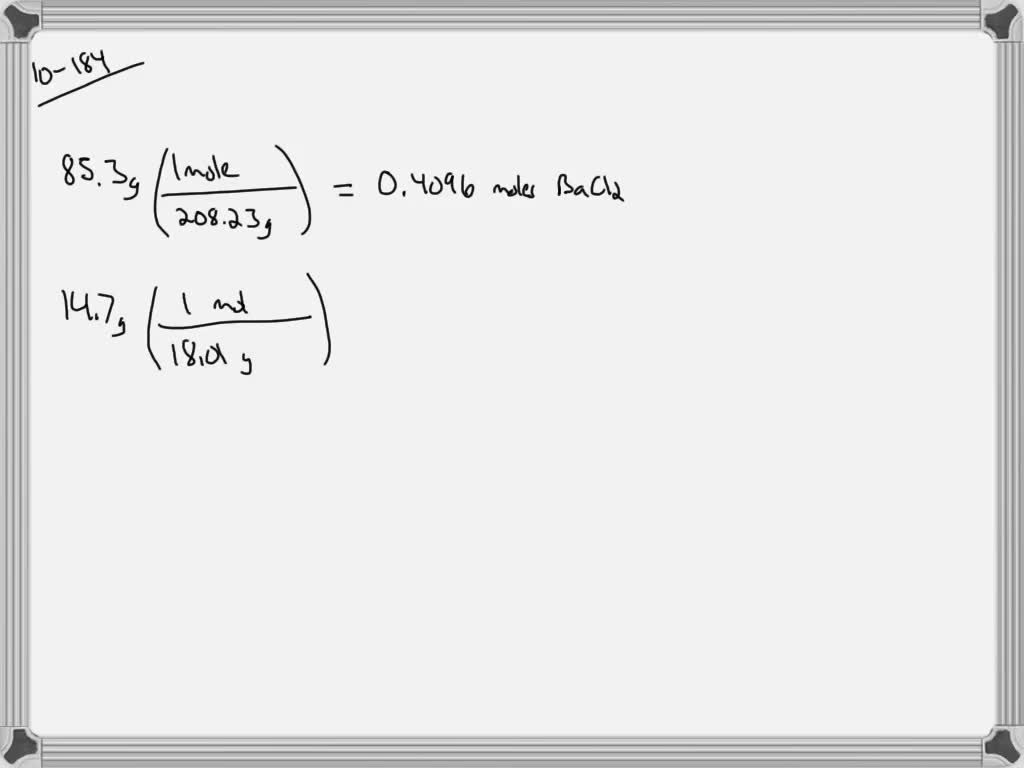
Three chromium(III) sulfates are well characterized:


 0 kommentar(er)
0 kommentar(er)
